Research Areas
With the constant ageing of the society our quality of life depends increasingly in our capability to regenerate damaged or phatological tissues and organs. At BioSmarTE we focus on the regeneration of complex tissues, trying to give a solution to those who suffer from conditions and pathologies that have yet not found a definitive cure. Our main approach to target this goal is through the combination of advanced smart materials and biofabrication techniques such as 3D printing (in all its modalities) and other additive manufacturing technologies.
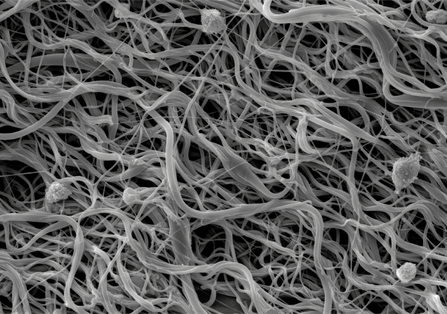
Biomimetic Hierarchical Scaffolds
Tissues and organs account for a hierarchical structure that determines their functionality. Cells interact with each other and their microenvironment via membrane receptors and acquire distinct morphologies (e.g., spindle vs rounded) and conformations (e.g., aligned vs random or layered vs aggregated) depending on the sensed stiffness and composition. Further, the extracellular matrix of tissues can be assembled into multiple well differentiated layers that define their mechanical properties. Altogether, these properties define the fate of the cell, the composition of the matrix and ultimately the functionality of the tissue.
Actuating Scaffolds
Tissues are constantly subjected to external stimuli that can be mechanical, electrical or chemical. These stimuli are dynamic input signals that are transmitted to the cell nucleus and translated into responses affecting their survival, proliferation, migration, differentiation, and more. Thus, controlling the external signals that cells sense allows us to control their fate and, hence, the kind of tissue they produce.
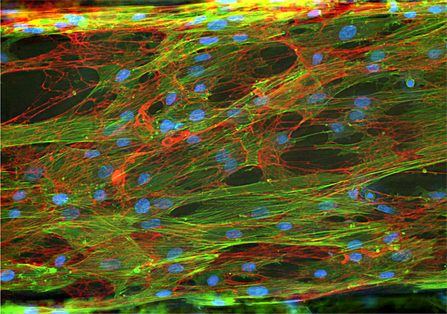
(Multi)Cellular Models
The development of treatments and strategies to heal tissues and organs (pathologic or not) requires the understanding of the tissue cellular microenvironment. Moreover, the validation of these strategies is a fundamental step towards clinical translation. The development of realistic (multi)cellular models of organs and their microenvironment will allow reducing the use of animal experimentation as well as to understand the signalling and interplay between different cells in pathologic tissues.
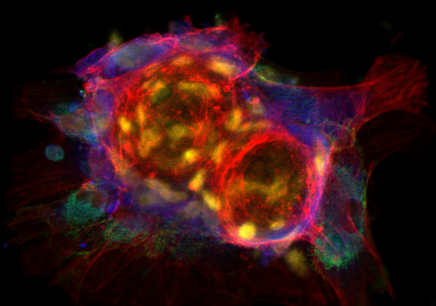
Immunoregeneration
The implantation of a foreign body into a patient results on the activation of an innate immune response. This response is initiated by macrophages and, after an initial inflammatory state can be either resolved or sustained in the time leading to implant rejection. Macrophages contribute to the process by expressing and releasing a series of cytokines that can be considered as pro-inflammatory (M1 phenotype)or pro-regenerative (M2 phenotype) orchestrating the immune response. Thus, it becomes essential to design implant materials that can guide macrophage polarization towards a pro-regenerative phenotype that enables tissue formation.
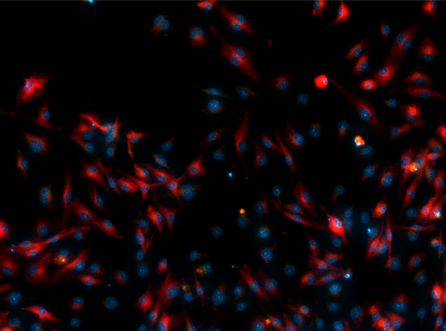
Biofabrication
Biofabrication technologies, in particular additive manufacturing or 3D bioprinting emerged over a decade ago with the promise of providing fast, reliable and user centred platforms for the fabrication of implant scaffolds that would give a definitive solution to tissue regeneration. Since then, there has been a boom on the knowledge and strategies generated, coming closer to the development of effective implants. However, these techniques generally lack of control of surface features and spatial composition.